
The most prominent protein component on the viral surface is the S glycoprotein – a large transmembrane protein that assembles into trimers to form the distinctive surface spikes of coronaviruses ( Walls et al., 2020 Tang et al., 2020). SARS-CoV-2 is an enveloped, single-stranded RNA virus of the family Coronaviridae its genome encodes 16 nonstructural proteins and four structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N) ( Naqvi et al., 2020). In December 2019 a novel coronavirus emerged ( Chan et al., 2020), named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the causative agent of the disease, COVID-19 ( Wu et al., 2020 World Health Organization, 2020). Therefore, the Elecsys Anti-SARS-CoV-2 S assay demonstrated a reliable performance across various sample populations for the detection of anti-S antibodies. The Elecsys Anti-SARS-CoV-2 S immunoassay had significantly higher sensitivity (≥14 days post-PCR) compared with the ARCHITECT SARS-CoV-2 IgG (98.70% vs 87.01% ), iFlash-SARS-CoV-2 IgG (100.00% vs 93.42% ) and IgM (100.00% vs 35.53% ), and EUROIMMUN Anti-SARS-CoV-2 IgG (98.26% vs 93.91% ) assays. For specificity and sensitivity analyses, 7880 presumed negative pre-pandemic samples and 827 SARS-CoV-2 PCR-confirmed single or sequential samples from 272 different patients were tested, respectively. We evaluated the assay performance using samples from seven sites in Germany, Austria, and Switzerland. SARS-CoV-2 Antibodies (NCVIGG, NCVIGQ), The qualitative detection of anti-Nucleocapsid IgG (NCVIGG) and the quantitative detection of anti-Spike IgG (NCVIGQ) antibodies.The Elecsys® Anti-SARS-CoV-2 S immunoassay (Roche Diagnostics International Ltd, Rotkreuz, Switzerland) has been developed for the detection of antibodies to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S) protein.

are able to perform blood draws for testing with a valid provider order. We recommend outside providers arrange to have their patients' blood drawn at their usual clinical draw sites and sent to the lab, preferably after contacting Client Support Services at to facilitate testing.įor patients who do not regularly seek care within UW Medicine, our phlebotomists at the University of Washington Medical Center-Northwest Campus (UWMC-NW) and UWMC-NW Outpatient Medical Center (OPMC) located on Meridian Ave. Ordering: We are pleased to perform serology testing for all patients who have a valid provider order. Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
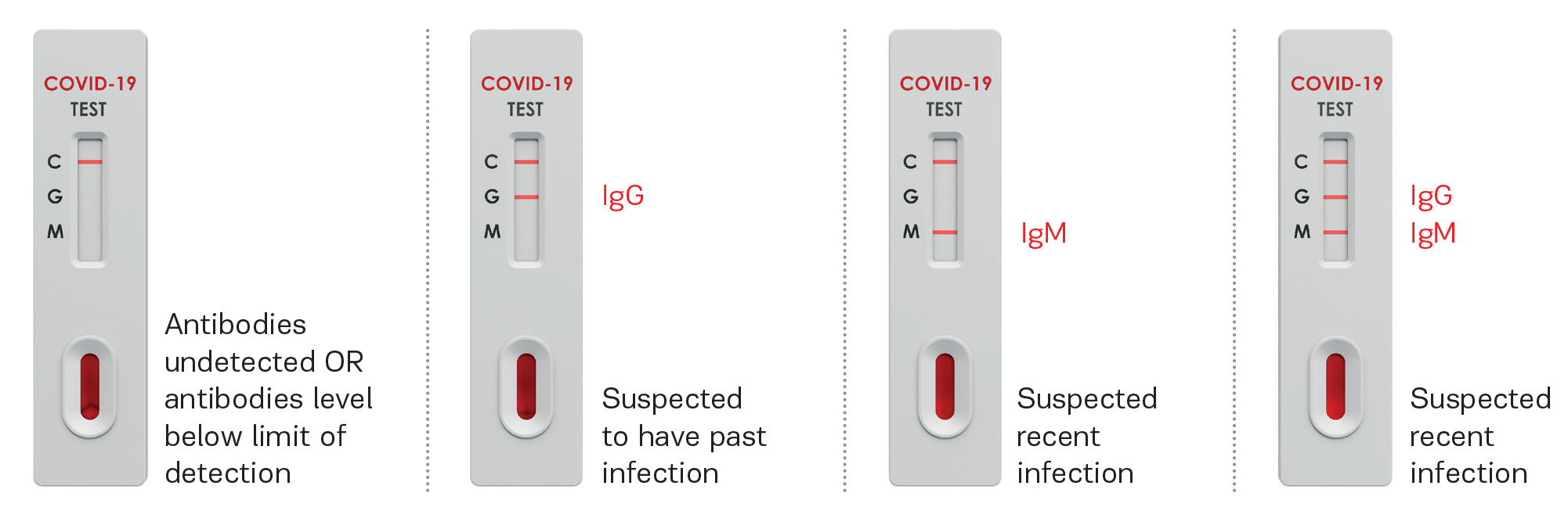
Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals. Nonreactive (Negative, <50.0 AU/mL) results do not rule out SARS-CoV-2 infection, particularly in those who have recently been in contact with the virus. Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.Ī table of quantitative anti-spike levels for otherwise healthy, recently vaccinated individuals by week of vaccination to aid in interpretation of test results is available in Table 3 in this pre-print. Reactive (Positive, ≥50.0 AU/mL) results may be due to immunization or past or present infection with SARS-CoV-2. The Abbott Architect SARS-CoV-2 IgG II assay, run under an emergency use authorization from the FDA, is quantitative test designed to detect IgG antibodies to the spike protein of SARS-CoV-2 in serum and plasma from individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection.


 0 kommentar(er)
0 kommentar(er)
